Abstract

Background:
Although survival of adolescent and young adults (AYA) with acute lymphoblastic leukemia (ALL) has improved using pediatric-inspired protocols, the outcomes of those who relapse remain poor using adult chemotherapy salvage-regimens that have historically incorporated high dose Ara-C based regimen, with long term-survival of less than 10%. The CR rate with blinatumomab for salvage in adult ALL is 34% with a median survival of 7.7 months, with an OS of less than 30% at 18 months. In contrast, pediatric salvage regimens have historically been based on multi-agent therapy and have reported superior outcomes. The ALLR3 protocol for relapsed ALL in patients up to the age of 18 years reported a CR rate of 97% and an overall survival of 72%. We therefore aimed to analyze the outcomes of young adolescents and adults treated on the R3 regimen +/- blinatumomab.
Methods:
Seventeen AYA patients with Philadelphia-negative ALL aged between 14 and 40 years who relapsed after first-line therapy and treated with ALLR3-protocol were identified. Relapses were classed as very early (<18 months from diagnosis) early (>18m from diagnosis and <6 months from end of therapy) and late (>6 months from end of therapy). The protocol consisted of vincristine (1.5mg/m2 IV D3,10,17,24); mitoxantrone ( 10mg/m2 IV d1 &2); PEG-asparaginase (1000 u/m2 IV d3 & 17); dexamethasone (10mg/m2 BID day d1-5 and d15-20); and intrathecal methotrexate (12mg d1 & d8) were identified. Patient aged 30-40 years received L-Asparaginase instead of PEG-Asparaginase, and 8mg/m2 of dexamethasone instead of 10mg/m2. Patients' characteristics, response rate (RR) and overall survival (OS) were analyzed. Patients who achieved minimal residual disease (MRD) negative CR proceeded with an allogeneic transplant. MRD-positive patients received blinatumomab followed by a transplant.
Results:
The median age at relapse was 16 years (14-40 years), 88.2% of patients were B-cell ALL and 11.8% were T-cell ALL. Most patient had standard cytogenetics on presentation (76.5%) and were CNS negative (82.4%). Relapses were very early, early or late in 2 (12%) , 5 (29%) , and 7 (41%) of cases, respectively. Relapses were isolated bone marrow (BM), isolated extramedullary (EMD) or combined BM and EMD in 14 (82%),1 (6%) and 2(12%) cases, respectively.
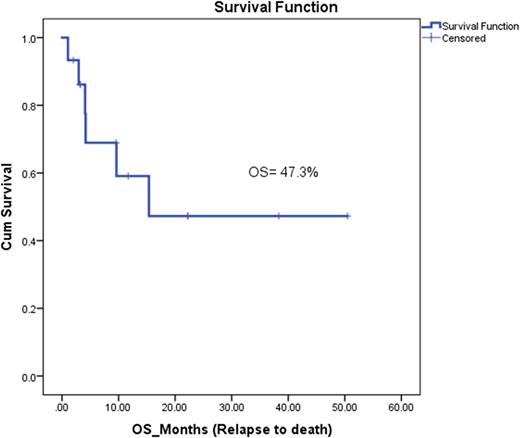
Overall complete remission (CR) rate was 60% at the end of R3 induction; CR with negative minimal residual disease (MRD negative, <10−4 cells) at the end of R3-induction was achieved in 47.1% of patients, while 11.8% of patients had CR with positive MRD (≥10−4 cells) who received Blinatumumab thereafter with eradication of MRD. Refractory disease was seen in 41.2% of patients. Almost all patients who responded to R3 proceeded to allogeneic stem cell transplant (88.8%). Eight patients died (47%), but only one patient died because of treatment-related sepsis, while the other 7 patients died because of disease-related causes. At a median follow up of 4 years, OS was 47.3%.
Conclusion:
Compared to traditional adult-based chemotherapy regimens and antibody therapies used in relapsed ALL, the use of pediatric-inspired protocol (R3) in relapsed AYA-ALL patients showed favorable response rates and overall survival. Further trials are required of pediatric inspired salvage regimens, preferably incorporating antibody therapies in adult patients, especially in 1st relapse.
Disclosures
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal